Radioactive
By Roger Russell
This
page is copyrighted.
No portion of this site may be reproduced in whole or in part
without written permission of the author.

![]()
What is on these Pages?
|
The Radium
Fad Is Radium
Paint a Danger Today? |
![]()
 Radioactivity occurs in unstable
elements. Including variations, at least 76 have been identified. These
elements go through the process of decay. Depending on the type of decay, they
can emit alpha particles (helium nuclei), beta particles (electrons) and gamma
rays (shorter than x-rays) at very high velocities. (The word radioactive is
misleading and actually has nothing to do with radio waves)
Radioactivity occurs in unstable
elements. Including variations, at least 76 have been identified. These
elements go through the process of decay. Depending on the type of decay, they
can emit alpha particles (helium nuclei), beta particles (electrons) and gamma
rays (shorter than x-rays) at very high velocities. (The word radioactive is
misleading and actually has nothing to do with radio waves)
One of the most widely known radioactive substances found in nature is uranium.. We have all heard of the need to process uranium ore and concentrate it for use in power reactors or bombs. Uranium ore is typically very colorful as shown in the left photo. Uranium 238 (U238) continually decays into 15 other elements, including the intermediate elements of radium and radon gas, eventually ending up as an isotope of lead 206 (Pb206).
Radioactive materials are often referred to in terms of half-life. This is the time it takes for half of the material to decay. Although the half-life of U238 is 4.5 billion years, other steps in the process take only a fraction of a second. Radium 226 (Ra226) has one of the longer steps and has a half-life of about 1600 years.
Becquerel, a French physicist, found that uranium would affect a photographic plate. His associates, Pierre and Marie Curie, found that an ore called pitchblende had a higher activity than uranium oxide. In 1898, they were able to extract a minute quantity of a new element four million times as active as uranium oxide. They called it radium. It takes about 15 tons of ore to produce one gram of radium in the form of a salt.
![]()

Uranium has been used as far back as Roman times to produce yellow colors for mosaic tiles and glass. More recently, it has been used in a wide variety of glass objects such as jars, ashtrays, dishes, vases and even children's marbles. Uranium glass has been referred to as yellow glass, canary glass, Depression glass and Vaseline glass. This can have a transparent greenish, yellowish or pink color. This kind of glass exhibits a bright greenish-yellow or green when exposed to long wave ultraviolet light. Even in open shade as shown above the 5/8" marbles have an unusual brilliance. They contain uranium. The radiation measures only 0.28 mR/Hr with a Geiger counter right up against one of them and they are completely safe.
![]()

 Here is a specimen from a leg bone
fossil shown at the left. It is from the Morison Formation in the Colorado
Plateau. It was part of a living animal during the Jurassic period around 144
to 208 million years ago. This bone has calcite crystal cells with a reddish
brown cell wall matrix. The end has been cut to show the cross section. It
weighs 19 ounces and is 3.25” X 2.75” X 3”
Here is a specimen from a leg bone
fossil shown at the left. It is from the Morison Formation in the Colorado
Plateau. It was part of a living animal during the Jurassic period around 144
to 208 million years ago. This bone has calcite crystal cells with a reddish
brown cell wall matrix. The end has been cut to show the cross section. It
weighs 19 ounces and is 3.25” X 2.75” X 3”
This fossil is radioactive but not all fossils might be radioactive. One clue is that it can be very colorful and here it has orange and red colors. The amount of radioactivity depends on what minerals were in its surroundings when it was fossilized. Although the radiation is very minor in this bone sample, it is measurable with a Geiger counter placed against the cut face (about 0.13mr/hour). The same process can apply to teeth as well.
The Spinosaurus (Jurassic Park III) tooth at the right was found in Morocco at the fringe of the Sahara Desert and shows a much lower level of radioactivity. These pieces confirm what I had heard about dinosaur fossils being radioactive.
![]()
 The first atomic bomb detonation was on July 16, 1945
in Trinity, New Mexico. The intense heat formed a grayish-green, glass-like
material from the fireball and fused the desert sand into a jade green crater
2400 feet in diameter. Nearly 2000 tons of intensely radioactive material was
formed. It was mostly silica with traces of olivine and feldspar.
The first atomic bomb detonation was on July 16, 1945
in Trinity, New Mexico. The intense heat formed a grayish-green, glass-like
material from the fireball and fused the desert sand into a jade green crater
2400 feet in diameter. Nearly 2000 tons of intensely radioactive material was
formed. It was mostly silica with traces of olivine and feldspar.
As soon as soil radiation dropped to safe levels, Trinitite pieces began to disappear into the hands of collectors and visitors looking for mementos. In 1975, Trinity was declared a national historic site and removing Trinitite was strictly prohibited. This sample of Trinitite is still radioactive but is only in the range of 0.3 to 0.5 mR/hr and is harmless. It is 7/8” wide and weighs 3.3 grams.
![]()
Being a devoted fan of popular radio programs in the 1940s, one of the programs I regularly listened to was the Lone Ranger. The William Tell Overture became a familiar sound and could be heard at the beginning of each program. At the end of World War II, the secret was out about the atomic bomb. In 1947, the Lone Range program was sponsored by Kix cereal and advertised an atomic bomb ring. Wow! I had to have one of these and it was only 15 cents. You could actually see atoms split through a lens in the ring.

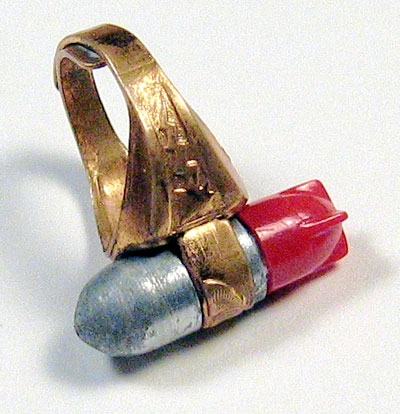
Although I don’t know what became of my original ring, I was able to find one later. Some of the silver-colored plating had been worn off the metal part but the red plastic fins were in good shape. The metal gold-colored ring portion was split and could be adjusted to fit different size fingers. A small piece of very thin paper for secret messages could be folded up and kept in the hollow fin section.

When the tail fin is removed, the viewing lens is exposed. Of course, this should only be done in the dark and after your eyes have adjusted to the dark.. Otherwise you will only see a steady green glow and no atomic action. Although the atomic action can no longer be seen after almost 65 years, the green glow can still be faintly seen after exposure to light.

![]()

After the success of General Mills and the Atomic Bomb Ring, Ralston offered a Space Patrol Ray Gun Ring. Like the Atomic Bomb Ring, this was also a spinthariscope with a radioactive screen. On the side of the ring can be seen a radioactive symbol, star, space ship, moon and Saturn.
![]()
United Nuclear Scientific Equipment and Supplies
The correct term for these rings is a spinthariscope. The radioactive material may have been polonium, a short–lived radioisotope. If you want to recapture those days, a spinthariscope is still available from United Nuclear.
|
|
|
Theses Spinthariscopes do not contain any dangerous Radium Bromide. Instead, they contain a tiny speck of extremely high grade Thorium ore, specially mined & imported from the Great Bear Lake in Canada. The high Thorium content makes this Canadian ore very unique in its chemical composition, and is the only natural occurring radioactive material that will put on the dazzling nuclear display you see in the Spinthariscope. The small speck of radioactive material is permanently sealed within the device and does not pose any health or radiation risk. The tiny radioactive source is encased in a metal tab and permanently bonded within the unit. The target material is a special "activated" blend of ZnS manufactured by United nuclear and sealed under clear plastic.
As the source material undergoes natural radioactive decay, atoms of it continuously explode, releasing Alpha Particles traveling at over 20,000 miles per hour. Even at this high speed Alpha particles can only travel a little over an inch in the air and can't even penetrate a sheet of paper. They can however hit the ZnS target suspended directly above the source. When an Alpha Particle hits the ZnS target, it releases a photon, the basic component of light. This produces the thousands of tiny flashes (scintillations) of blue-white light you see through the magnifying lens. Alpha radiation (Alpha particles) are just common Helium atoms that have lost their electrons and are traveling at very high speed. The Nuclear Spinthariscope features an adjustable focus, requires no batteries or any other form of energy, and will continue to operate producing tiny visible nuclear explosions for at least 60 years.
![]()
Tom Mix Magic Light Tiger-Eye Ring
|
|
|
The Tom Mix Magic-Light Tiger-Eye Ring used Polonium 210 to illuminate a zinc sulfide screen. This was not a spinthariscope but never-the-less was a radioactive source. It just glowed. A plastic lens over the screen resembled a cat’s eye. It was supposed to be bright enough to signal in the dark. The phosphor itself still glows dimly after being exposed to light but the polonium has long since decayed.
![]()

I had been collecting WWII military patches since the 1940s. There were a few stores along Broadway in New York City that had all kinds of patches mounted on boards on the wall. Every time we went to the city, my mother would buy a few for me to add to my collection. The colors were fascinating to look at. This was the US Army Manhattan Atomic Project patch.
![]()
Today, the radium paint has deteriorated on the hands and numerals of this Jefferson Golden Minute clock. I have used a long-wave ultraviolet light to show the green color of the phosphor that could have been seen at night. It would normally glow all the time and did not need any external source of energy to do this. In daylight, the paint typically had a slight greenish color.
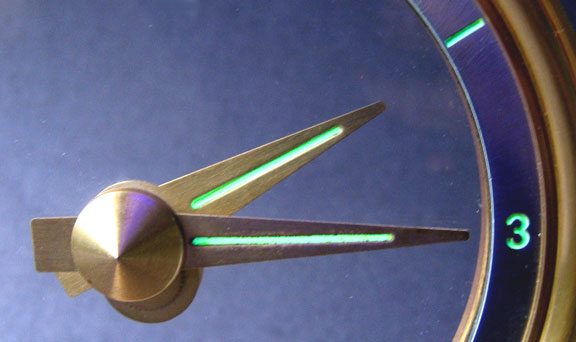
Radium paint was used on watches, clocks, aircraft instruments, etc. for many years until its use was banned in the 1960's. Manufacturing posed a serious health hazard. At the outset, very little was known about its effects on people but was later found to be a very serious health hazard for those who worked with it. One of the characteristics of the radium paint that was applied many years ago is that today it glows only very faintly or not at all. Since radium has such a long half life, that does not account for the change. It is the zinc sulfide (ZnS) that has changed. ZnS by itself will not emit light when bombarded with alpha particles. It must be intimately bonded. The original ZnS was activated by heating to 1500 degrees in the presence of silver salts and changed to a crystalline form. This type of ZnS will emit photons (light) when hit by alpha particles and also have a long afterglow. However, over a long period of time, continuous bombardment by alpha particles emitted by the radium destroyed the internal crystal structure. This has been verified by comparison using electron microscope images. Fractures in the crystals inhibit electron migration and the release of light.
I had hoped that if ordinary ZnS were applied to the old radium paint, it might immediately rejuvenate and would continue to glow for many more years. However, there appears to be more to the original process than was expected. If the ZnS is separated from the radium by the thickness of the binder, such as varnish, that is sufficient to prevent the alpha particles emitted from the radium from reaching the ZnS. Yet another process was required in the original paint manufacture to first bond the radium and ZnS intimately together and then to be held in place by the binder. Even painting a coat of activated ZnS on old radium paint does not provide close enough contact with the radium to increase brightness.
The original silver activated ZnS is no longer manufactured. The activated ZnS made today is quenched with copper in the manufacturing process to give very short duration light emission for use in radiation detection equipment. United Nuclear, Sandia Park, New Mexico is one of the few places that make the copper-quenched version.
Radium 226 was most commonly used in the paint and was a good alpha particle emitter. One of the best kept trade secrets of the various paint manufacturers was how they kept the brightness of their paint from decreasing. Brightness could be controlled with the amount of radium and the concentration of ZnS crystals. In addition, mesothorium, also known as radium 228, was sometimes added to the paint. This was a good beta emitter but had a half-life of only 5.8 years and decayed into thorium-22, an alpha emitter, with a half life of 1.9 years. The result was that the light intensity from the paint would increase over the first 5 years and then decrease with the normal ZnS crystal deterioration. Radium paint, of course, is no longer available.
![]()
Radium paint was very popular in the 1920's and even before. Although one of the most popular uses for radium paint was for watches and clocks, an incredible number of other uses appeared as well. One of these was radium water. This was particularly popular in Europe in the 1920’s and many different companies were making these “vitalizers” that contained water charged with radon gas. Radium was even used by a few people to spread on their gardens to supposedly enhance them.
There were radium injections, pills to be taken orally, creams, ointments, lipstick and even suppositories. Although the medical profession challenged the claimed benefits for these “quack” treatments, its use continued until the 1960’s. I was personally involved at the age of nine when I had my tonsils and adenoids removed in the mid 1940’s. I was given several radium treatments that were to prevent any growth return. Radium tipped rods that were otherwise stored in a lead container, were inserted way in my nose for 10 or 15 minute periods. Fortunately, many years later and after several medical tests, no ill effects have appeared. However, not everyone was that lucky, particularly if they were exposed at an earlier age.
One company that manufactured radium for use in various products was the Radium Luminous Material Corporation, 58 Pine Street, New York City. The factory was in Orange, NJ and the mines were in Colorado and Utah. A full-page ad was in The Literary Digest, November 13, 1920 for radium products on page 65. It is called UNDARK (trade name applied for). It was recommended for all kinds of uses such as watches, clocks, doorbell buttons, fish bait, gauges, theater-seat numbers, fire extinguishers and poison labels. A "try-out" set was available for $3.00 through the mail. The ad is shown in Figure 6.

![]()

Radium dial painting began in 1917 but it was eventually known to be deadly for the dial painters. Young women ranging in age from the mid teens to the early 20's were employed to apply the paint to clock dials and other products for several different companies. The dial painters were typically single and lived with their parents. Dial painting was enjoyable work with comparatively high wages. Working conditions were excellent and the rooms were well illuminated. Over the first 10 years, about 2000 women were employed in this work, mostly in three locations: Orange, NJ, Waterbury, CT and Ottawa, IL. This picture of the dial painters was taken at the Ottawa Radium Dial Company in 1924.
The earliest dial painters had come from the china-painting industry. Workers were required to place the tip of the brush between their lips to bring the paintbrushes to a point. This was called “lip-pointing.” It was the best way to accomplish the very fine work. The practice was passed on to the radium-painting industry that also needed fine work. After a successful one-month trial period, new employees were given a permanent position. The remainder was dismissed. New workers initially painted clock faces and hands. If their work improved, they were given smaller items such as alarm clock or pocket watches. Weekly earnings varied from $17.00 to $42.00 depending on how many pieces were completed.
 The painting was done exceptionally well. A typical
painted dial is shown on the Trojan watch at the left. Although some of the
paint has flaked off over the years, the careful work of applying the radium
paint can still be seen. The radium dial of this old watch could have been
painted by a dial painter who later may have suffered and/or died as a result
of imbibing radium paint while painting the numbers and hands on this and many
other watch dials.
The painting was done exceptionally well. A typical
painted dial is shown on the Trojan watch at the left. Although some of the
paint has flaked off over the years, the careful work of applying the radium
paint can still be seen. The radium dial of this old watch could have been
painted by a dial painter who later may have suffered and/or died as a result
of imbibing radium paint while painting the numbers and hands on this and many
other watch dials.
By the 1920's and 1930's, some dial painters and former dial painters began to suffer from a variety of illnesses, often crippling and frequently fatal as a result of ingesting the radium paint. Ingested radium is known to deposit permanently in bone structures. Radiation can then damage bone marrow, causing anemia. It can also weaken the bones so they might crush or snap under normal pressure. It can weaken bone tissue making it easy to get infection such as the jawbones that have dental work or gum disease. It can cause other forms of cancer in the sinus and mastoids.
The dial painters were some of the first victims of radioactive poisoning. Earlier, Marie Curie, who first discovered radium in 1898, died from leukemia, which in all probability was caused by her long exposure to radium. Sabin Von Sochocky discovered a luminous paint formula that included radium and founded a company that sold luminous clock and watch dials. He died from aplastic anemia, also likely caused by radium exposure.
It isn't clear how well known the dangers of radium were in 1917 but no warning was given to the workers. The radium companies denied the dangers of ingesting radium despite the consensus of opinion among most medical experts and government officials that it was dangerous. The dial painters were a minority and lacked any financial resources to be effective in dealing with industry. The battle for recognition of this health hazard to these women went on for many years.
Although the radium-painted watches and other items were sold by various companies, it is not known if any were painted by their own employees. For instance: the Ottawa facility painted all of the radium dials used by Westclox. About 4,300 dials were painted per day. The use of radium paint was eventually banned in the 1960’s
A book titled Radium Girls: Women and Industrial health reform, 1910-1935 by Claudia Clark was published in 1997. This is an excellent source of information on the subject. It is well documented with many references and an extensive bibliography. However, there are no pictures. Another book titled Deadly Glow: The Radiom Dial Worker Tragedy by Ross Mullnerm PhD, MPH has an excellent account of the dial painters and has many pictures.
|
|
|
A 1-3/4 hour documentary titled "Radium City" was made in 1986 about the after effects of two radium dial painting companies based in Ottawa, Illinois. The city of Ottawa, IL is about 80 miles southwest of Chicago. The Radium Dial Company (RDC) moved from the east coast to Ottawa in 1922. Joseph Kelly was President. The first problems of radiation exposure occurred with the young women who applied the radium paint to the dials. According to the film, RDC went out of business in 1934 after being faced by many lawsuits. Luminous Process Incorporated (LPI) went into business soon after and was also headed by Kelly. It operated from 1932 to 1978 when the NRC shut it down. Both factories were demolished from 1984 to 1986 and much of the material was used as landfill. As a result, there are 13 areas today with above normal radiation in Ottawa. The major contaminant is radium-226 and the byproduct, radon-222. A video tape of this documentary is available on loan to members of the NAWCC and can be borrowed from the National Watch and Clock Museum Library and Research Center. For more information see the Petitioned Public Health Assessment, Ottawa Radiation Areas, Ottawa, Lasalle County, Illinois
![]()
Is Radium Paint a Danger Today?
 Unfortunately, there is not a simple
answer when it comes to radiation exposure to the paint. Opinions vary widely
about how dangerous old radium watches and clocks are. The explanation is more
difficult to understand than just risk or no risk. There are all degrees of
risk in between. Of course, zero exposure can be considered the best of
conditions but we are all exposed to a small amount of radiation each day from
cosmic rays, radioactive elements in the air, food and water.
Unfortunately, there is not a simple
answer when it comes to radiation exposure to the paint. Opinions vary widely
about how dangerous old radium watches and clocks are. The explanation is more
difficult to understand than just risk or no risk. There are all degrees of
risk in between. Of course, zero exposure can be considered the best of
conditions but we are all exposed to a small amount of radiation each day from
cosmic rays, radioactive elements in the air, food and water.
Radiation can be measured in units called roentgens. Because many sources emit only a fraction of this amount, measurements are normally referred to in units of milliroentgens (mR) or one one thousandth of a roentgen. The length of exposure is also important and so the radiation is also referred to in terms of time, such as mR per hour or mR per year. A Geiger counter, such as the one shown in Figure 4, can be used to measure the amount of radiation from radioactive sources such as this Jefferson Electric “Golden Minute.” This highly accurate nuclear radiation monitor is made by International Medcom, 6871 Abbott Ave., Sebastopol, CA 95472. This particular model is called the "Inspector."
Radiation strikes the sensitive circular detector tube in the back and causes an avalanche of electrons. This is amplified and can be seen on a digital readout or heard as a click through the built-in speaker. The higher the radiation, the higher the reading and the faster the clicks occur. With the Geiger counter away from any radioactive sources, background radiation in my home averages about 0.003 mR/hr.
Early Jefferson Golden Hour, Golden Minute and Golden View clocks have luminescent paint applied to the hands and numerals on the dials. Jefferson sales literature mentions that radium is used. Measurements made recently on these old clocks, using a Geiger counter, show the levels to be extremely low and are NOT considered a hazard. On contact with the minute hand the dose rate is 1 mR/hour. At a distance of one foot, the dose rate is about 0.008 mR/hour. This is about the same level found for old radium dial wristwatches. Production of the Golden Hour began in December of 1949 and luminescent paint has been found on Golden Hours as late as 1965.
According to the National Council on Radiation Protection and Measurements, the continuous or frequent annual external radiation exposure to a population should not exceed 100 millirem per year. A limit of 500 millirem per year for an individual should be applied for an infrequent annual exposure. This does not mean that the total for a year can all occur within a short time of a few seconds but rather is spread out over a one-year period. It should be pointed out that exposure is cumulative. If you are exposed to radiation of 1 mR/hr for 3 consecutive hours, your total exposure for that time is 3 mR.
The exposure to clocks with radium dials and hands, such as the Jefferson Electric Golden Hour, Golden Minute and Golden View clocks, is normally not considered a risk because those clocks are not kept close to the body. The maximum level is 0.20 mR/hr at one foot (1) and decreases rapidly with distance. However, there is no cover to protect from flaking paint or radon gas that again is at a very low level..
The only real danger with radium paint is when attempts are made to remove it. Some authorities say that even removing a watch dial could result in breathing any accumulation of radon gas or radioactive dust that has come free. Scraping it off with a knife blade and letting the pieces get all over could result in later ingesting some and then it could be a health hazard for you, your family and pets. Then, there is the problem of getting rid of the pieces. Wherever you put them, they are still a radioactive hazard. If you work with radium dials frequently, you should become more familiar with the hazards and precautions of working with radioactive materials.
Disposal of radioactive materials may vary from state to state, so it would be best to check with your local Environmental Protection Agency for proper disposal. In some cases, your local EPA may accept a radium dial watch at no charge. Otherwise, a broker may be required to dispose of any quantities of radioactive material. Aircraft instrument dials and knobs are frequently disposed of in this way. Fees can run around $1000 and the items are picked up at your location. A well-known broker is Bionomics Incorporated, PO Box 817, Kingston, TN 37763. They are licensed and have permits to remove, package, transport and dispose of radioactive materials.
If shipping watches or clocks by mail, check with the postal regulations regarding radioactive substances. The rules, if complied with, are fairly lenient and should not be a problem for shipping watches or clocks with radium paint.
![]()
 Today, a new material is used in wrist watches,
aircraft instruments, and the military, etc. This is a radioactive isotope of
hydrogen called tritium. It originally had been used to increase the yield of
atomic weapons. Tritium is a gas and has a half-life of 12.43 years. It decays
into a stable, non-radioactive form. In commercial use, the gas is put in tiny
vials of borosilicate glass that are internally coated with phosphor and sealed
by a laser.
Today, a new material is used in wrist watches,
aircraft instruments, and the military, etc. This is a radioactive isotope of
hydrogen called tritium. It originally had been used to increase the yield of
atomic weapons. Tritium is a gas and has a half-life of 12.43 years. It decays
into a stable, non-radioactive form. In commercial use, the gas is put in tiny
vials of borosilicate glass that are internally coated with phosphor and sealed
by a laser.
These assemblies are referred to as radio-luminescent light sources, also known as Gaslights or Trasers. The vials can be made so small that they can be used in watches. They can also be made large to be used as emergency exit signs for buildings, etc. Unlike radium paint, tritium gas emits only low energy beta particles (electrons) that are unable to penetrate the borosilicate glass vials. This makes their use entirely safe. The only risk with tritium is if the vials are broken but it is still a very low risk. However, because the gas is radioactive, there are more rules and regulations involved and importing has become essentially restricted for individual purchases.
The non-radioactive phosphors used today must be exposed to light to be effective but gradually fade away in the dark. Trasers glow constantly by themselves, even in total darkness and require no energy source other than the radioactive gas. Trasers are commonly made with green and yellow phosphors that can most easily be seen. Other colors include orange, red and blue. This watch is sold by mb-microtek, Switzerland. Trasers are used to indicate the hours as well as on the hands. They can be made so small that one can even fit on the second hand. There is no measurable radiation from this watch, even with the Geiger counter right against the crystal.
![]()
(1) Roger Russell “The ‘Dutch Secret’ and Jefferson Electric Clock History,” NAWCC Bulletin (October 2002: pp. 600-605.)
(2) Paul Frame and William Kolb “Living with Radium: The first Hundred Years” copyright William M. Kolb and Paul Frame, 2002. Published by Syntec, Inc.
(3) Claudia Clark, “Radium Girls: Women and Industrial Health Reform, 1910-1935” by the University of North Carolina press, Chapel Hill and London in 1997 (ISBN 0-8078-2331-7 cloth and ISBN 0-8078-4640-6 paperback.) Reprinted with permission from the University of North Carolina Press.
(4) Ross Mullner, PhD, MPH “Deadly Glow: The Radium Dial Worker Tragedy” copyright 1999. Reprinted with permission from the American Public Health Association.
(5) Roger Russell “Radium and the Dial Painters” NAWCC Bulletin October 2005. pp. 577-582
![]()
|
About This Site |
||
|
|
|
More text and pictures will be added as my research on this speaker continues. Any comments are welcome. |
|
|
|
Created
by Roger Russell |





